Selection and Combination of Low-Temperature Sintering Aids for Aluminum Oxide Ceramics
Aluminum oxide ceramics, known for their high insulation, thermal insulation, corrosion resistance, and high hardness, are widely used in various fields such as mechanical processing, electronics, chemical industry, and aerospace due to their extensive sources and low cost. However, the small atomic radius of Al2O3 crystals, strong ionic bonds, and high lattice energy require overcoming intense ionic bond interactions at very high temperatures (2050°C) to achieve melting and sintering. This not only consumes a considerable amount of energy and imposes high requirements on thermal equipment but also leads to abnormal grain growth at elevated temperatures, resulting in uneven structure and even large internal closed pores, which reduces the bonding strength between grains and decreases material performance. Therefore, research on low-temperature sintering technology for aluminum oxide ceramics is crucial, whether for reducing energy consumption, cost savings, or improving performance.
Currently, there are three main methods to lower the sintering temperature of aluminum oxide:
1、Reducing the particle size of aluminum oxide powder;
2、Adopting other advanced low-temperature sintering technologies;
3、Adding sintering aids.
Since using fine powders as raw materials is costly and advanced low-temperature sintering techniques such as microwave sintering and discharge plasma sintering have high process requirements and equipment costs, adding sintering aids directly to aluminum oxide powder materials is a cost-effective, efficient, and simple method compared to the former two. It is currently the most effective and feasible low-temperature sintering method.
Classification and Mechanism of Aluminum Oxide Low-Temperature Sintering Aids
The mechanism by which sintering aids promote densification of aluminum oxide ceramics is complex. According to the different roles of cations in various sintering aids in promoting sintering, they can be roughly divided into four types: formation of solid solutions with aluminum oxide, formation of eutectic systems with aluminum oxide during sintering, formation of new phases with aluminum oxide, and promotion of liquid-phase sintering due to the presence of low-melting glass phases in the raw materials.
1、Formation of Solid Solutions with Aluminum Oxide
The substitutional solid solution between sintering aids and aluminum oxide is an important mechanism for promoting the densification of aluminum oxide sintering. When sintering aids and aluminum oxide undergo solid solution substitution at high temperatures, solute atoms will replace solvent atoms in the lattice. The difference in ionic radius between the cation and Al3+ will cause lattice distortion and generate lattice defects. Moreover, due to the difference in the valence state between the cation and Al2O3, the tendency of the crystal to neutrality results in the formation of cation vacancies in the crystal, causing lattice contraction. These lattice defects and cation vacancies facilitate lattice activation, increase diffusion rates, and make aluminum oxide ceramics easier to recrystallize, thereby promoting sintering and reducing sintering temperature. Typically, sintering aids that can form solid solutions with aluminum oxide are oxides with lattice constants close to Al2O3, mostly containing variable-valence elements such as TiO2, Cr2O3, Fe2O3, and MnO2.
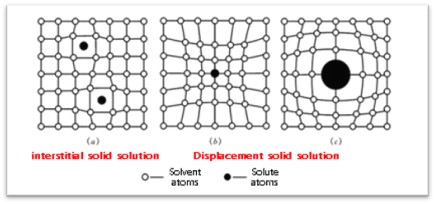
Lattice defects caused by solid solution replacement
2、Formation of Eutectic Systems with Aluminum Oxide
During Sintering Low eutectic solvents are eutectic mixtures formed by two or more solids through hydrogen bonding. Due to the strong interaction between the anions of the hydrogen bond acceptor (HBA) and the hydrogen bond donor (HBD) and the charge delocalization caused by the HBD hydrogen supply and the HBA component, their melting points are lower than those of each individual component. For example, the principle of sprinkling salt on snow utilizes the low eutectic principle to melt snow.
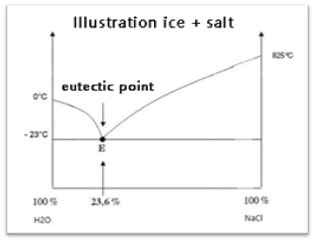
Typical application of ice + salt eutectic system
In the low-temperature sintering of aluminum oxide, sintering aids that can form binary, ternary, or multicomponent eutectic systems with other components, such as SiO2, CaO, MgO, SrO, and BaO, are added. When heated to the lowest eutectic temperature, a liquid phase begins to appear. Since the eutectic temperature is lower than the theoretical sintering temperature before the addition of the aid, low-temperature sintering is achieved.
3、Formation of New Phases with Aluminum Oxide
During Sintering During the sintering of aluminum oxide, some sintering aids (such as MgO, SiO2, etc.) added will undergo solid-phase reactions with aluminum oxide to form second phases such as magnesium aluminate spinel and mullite. The formation of second phases can activate the lattice, promote sintering of aluminum oxide, and improve the ceramic properties. Generally, the formation of second phases is often accompanied by the generation of other mechanisms such as liquid phases and solid solutions, playing an auxiliary role in promoting sintering. It is worth mentioning that the formation of new phases plays an important role in improving the properties of aluminum oxide ceramics. For example, the magnesium aluminum spinel formed on the surface of Al2O3 by MgO can reduce the interfacial energy, decrease the diffusion rate of grain boundaries, effectively inhibit the growth of Al2O3 crystals, and act as a stabilizer.
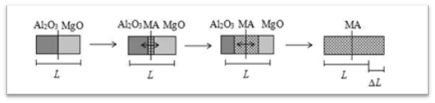
Schematic diagram of the reaction of alumina and magnesia to form magnesia-alumina spinel
4、Utilization of Low-Melting Glass Phases with Inherent Low Melting Points to Form Liquid Phases
During ceramic sintering, the introduction of low-melting glass phase substances (such as borates, etc.) with low melting points will gradually undergo a transition from solid phase to liquid phase with increasing temperature. The viscosity of the glass phase also changes. When the viscosity decreases to initiate viscous flow at a certain temperature due to capillary pressure, it can promote the rearrangement of powder particles in the green body, achieve a denser spatial stacking, and promote the dissolution of small particles or solid phase particles in the liquid phase. By liquid diffusion, condensation occurs on the surface of coarse particles, accelerating the reaction process, thereby achieving ceramic sintering at lower temperatures. Currently, the use of low-melting glass phase sintering aids can reduce the sintering temperature of aluminum oxide to around 900°C.
Principles for Selecting and Combining Sintering Aids
A single aid usually cannot simultaneously meet the requirements of sintering and mechanical and electrical properties. In some cases, the use of a single aid to lower the sintering temperature may lead to a decrease in ceramic performance. Therefore, in practical production, it is often necessary to use a combination of multiple aids to form composite additives.
To maximize the effectiveness of sintering aids without affecting material performance, the following principles should be followed when selecting composite aids:
.Different aids should have a synergistic effect on promoting sintering. Using multiple aids with different sintering mechanisms can better reduce the firing temperature compared to a single aid.
.Different aids should preferably not react with each other, as this may weaken or offset their sintering-promoting effects.
.Different aids can complement each other. The adverse effects of one aid on material properties during sintering promotion can be compensated by another aid.
When selecting different aids to form composite aids, glass formers such as SiO2 are mainly used as the main additives for forming low eutectic systems, supplemented by glass intermediate materials such as BeO and ZnO, and glass modifier materials such as MgO, Li2O, BaO, CaO, and Sr2O are used to form MgO-Al2O3-SiO2 (MAS), CaO-Al2O3-SiO2 (CAS), Li2O-Al2O3-SiO2 (LAS), and other sintering aid systems. For high-purity aluminum oxide ceramics, MgO is usually selected as the basic sintering aid to form magnesium aluminate spinel and construct low eutectic systems. However, the high-temperature volatility of MgO will cause large grains on the surface of the ceramic, which will affect the properties of aluminum oxide. Therefore, other sintering aids are needed to reduce the grain boundary growth rate of aluminum oxide, such as using MgO and La2O3 or Y2O3 in combination. Currently, it has been experimentally proven that commonly used composite aids such as the CaO-MgO-SiO2 system, the MnO2-TiO2-MgO system, and the CuO-SiO2 system can significantly reduce the sintering temperature while refining grains, stabilizing structure, and improving material mechanical properties.
XIAMEN MASCERA TECHNOLOGY CO., LTD. is a reputable and reliable supplier specializing in manufacturing and sales of technical ceramic parts. We provide custom production and high precision machining for a wide series of high performance ceramic materials including alumina ceramic, zirconia ceramic, silicon nitride, silicon carbide, boron nitride, aluminum nitride and machinable glass ceramic. Currently, our ceramic parts can be found in many industries like mechanical, chemical, medical, semiconductor, vehicle, electronic, metallurgy etc. Our mission is to provide the best quality ceramic parts for global users and it is a big pleasure to see our ceramic parts work efficiently in customers' specific applications. We can cooperate on both prototype and mass production, welcome to contact us if you have demands.





